Prior Research Accomplishments
After the initial derivation of induced pluripotent stem cells (iPSCs), concerns were raised regarding their functional equivalency to embryonic stem cells (ESCs). In response, we generated a large number of lines and characterized their genome-wide DNA methylation and expression profiles, and their ability to generate different neural cell types including postmitotic neurons (Boulting, Kiskinis et al., Nat Biotech; Bock, Kiskinis et al., Cell). We demonstrated that iPSCs are functionally equivalent to, and as variable as, ESCs. We also co-developed a novel quantitative method to assess pluripotency based on the expression of rationally selected, developmentally critical genes. This work has been cited more than 1200 times. Based on our findings, Life Tech developed a quality control assay for iPSCs (known as the Scorecard), which is widely used by the stem cell community.
We also developed some of the first iPSC-based cellular models of amyotrophic lateral sclerosis (ALS), an untreatable neurodegenerative disease that selectively targets motor neurons (Kiskinis et al., Cell Stem Cell; Wainger, Kiskinis et al., Cell Rep; Kiskinis et al., Stem Cell Reports). Using patient-specific iPSCs we uncovered a range of molecular pathways that lead to the selective degeneration of patient neurons. Our major finding was a previously unknown interaction between endoplasmic reticulum-related stress and electrophysiological defects that was mediated by impaired potassium current. This work led to a Phase II clinical trial with Ezogabine, a potassium channel agonist, which was recently completed, successfully meeting its clinical endpoints. Collectively, our work has contributed to the validation of iPSC technologies and provided one of the first examples of a discovery made using an iPSC model that was directly translated to the clinic.
Central Directions of the Lab
The lack of easy accessibility to the cells of the nervous system has hampered progress towards the discovery of degenerative mechanisms as well as more effective
treatments for neurological diseases. The groundbreaking technology of reprogramming, which allows for the generation of patient specific iPSCs has created an unprecedented opportunity for a new
approach towards developing cellular models of human disease. Pluripotent stem cells, which renew indefinite, retain the unique ability to generate every cell in the human body including the myriad
neuronal subtypes that make up the nervous system. Our research interests are focused on the following broad programs:
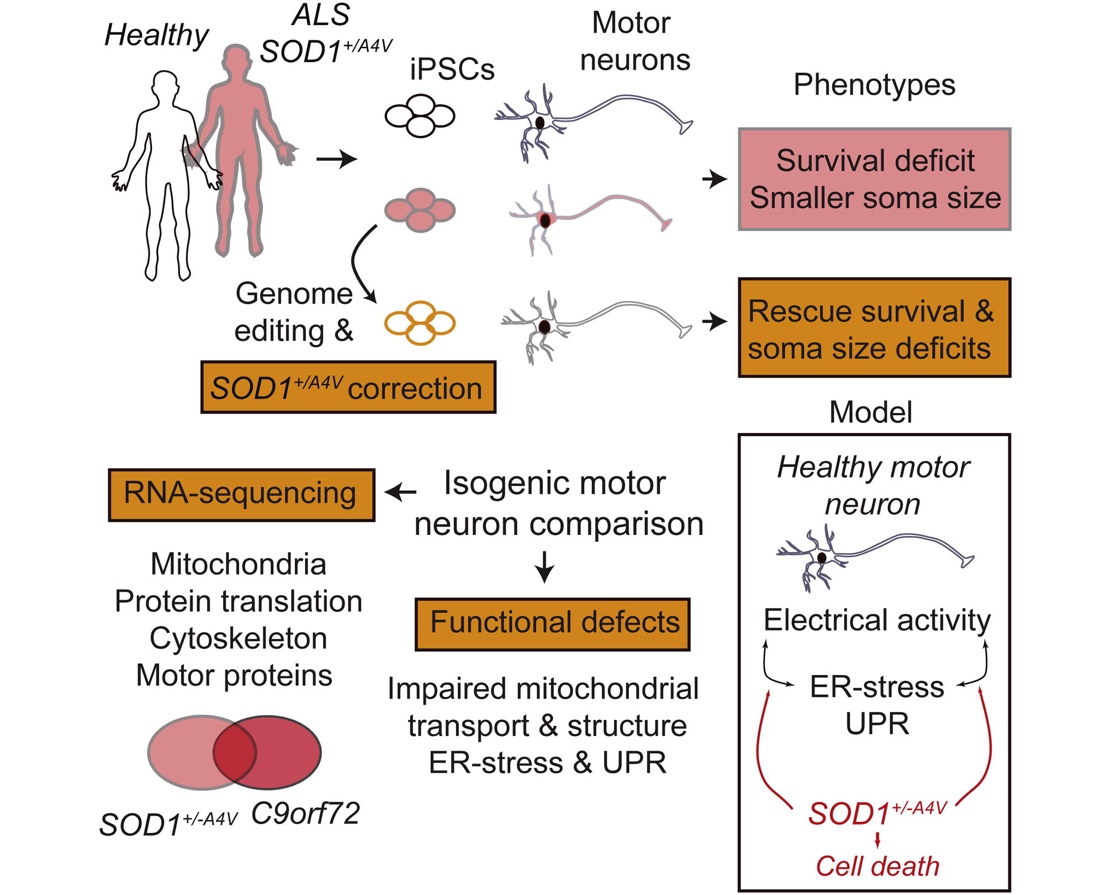
Uncovering the degenerative mechanisms that give rise to Amyotrophic Lateral Sclerosis
ALS is a clinically and genetically heterogenous disease characterized by the selective loss of cortical and motor neurons. While the majority of cases are sporadic, around 10% of ALS can be caused
by mutations in genes implicated in diverse set of cellular functions. Nevertheless, all patients are uniformly characterized by a common pattern of progressive degeneration with accumulating
neuropathological protein aggregates. This raises the possibility that different disease initiating events could coalesce in one or more common molecular pathways. To address this, we are generating
patient-specific iPSCs from genetic cases, using gene-editing approaches to fix the mutations and are analyzing the phenotypic alterations in diseased motor neurons. We are using these models
to discover disease mechanisms and illuminate broadly relevant routes for therapeutic intervention that may lead to rationally designed clinical trials and personalized treatments.
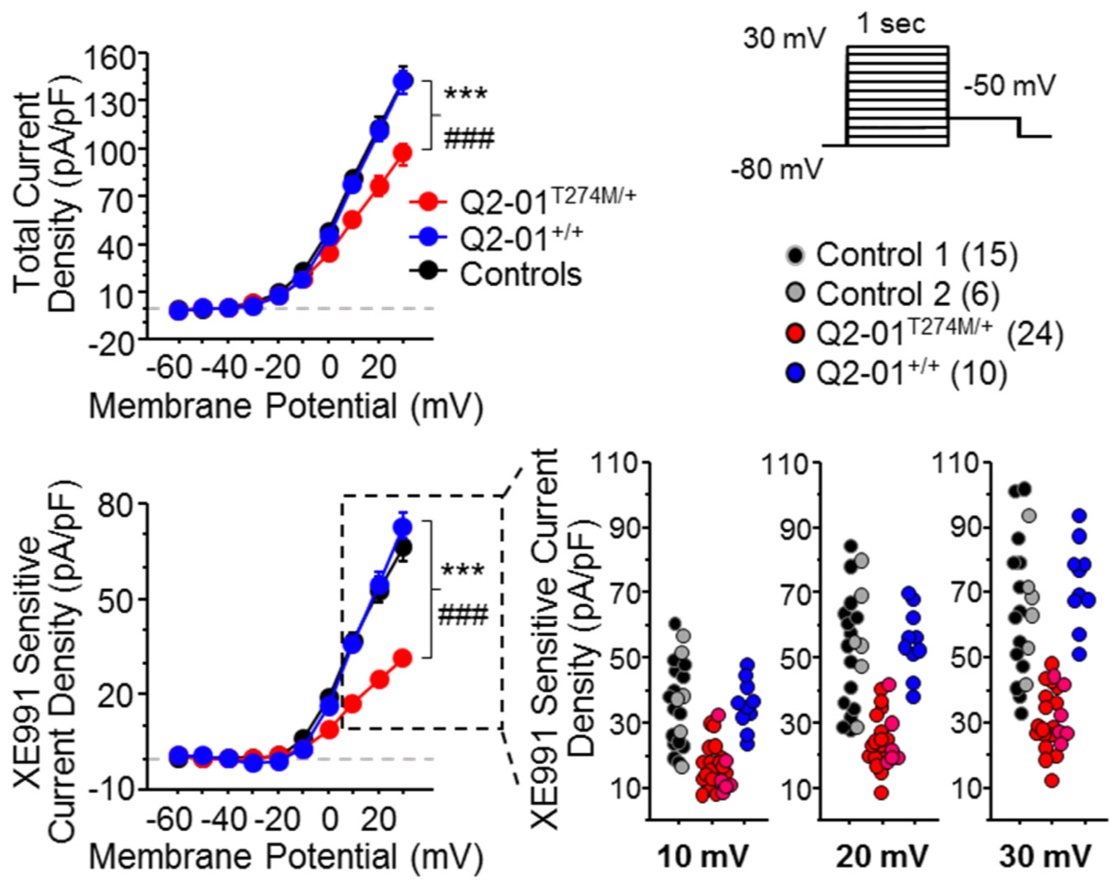
Developing iPSC-based models of epileptic channelopathies
Epileptic channelopathies are defined as seizure involving syndromes caused by mutations in ion channels such as KCNQ2 and SCN2A. Despite the availability of multiple anti-epileptic drugs,
the treatment of epilepsy remains inadequate. It is often unclear why a patient suffering from epilepsy does or does not respond to a specific drug treatment. We are focused on developing
cellular models of epileptic channelopathies using reprogramming and gene editing techniques (Simkin and Kiskinis, Epilepsy Curr; Simkin et al., eLife). We use a combination of high-throughput
electrophysiological assays including multi-electrode array (MEA) systems and the Optopatch, which allows for neuronal recordings with single cell resolution in an all-optical fashion.
Our goals are to gain insights into the pathophysiological processes that give rise to epilepsy, discover novel therapeutics and determine whether we can predict pharmacological
responses of patients by studying their neurons in vitro.
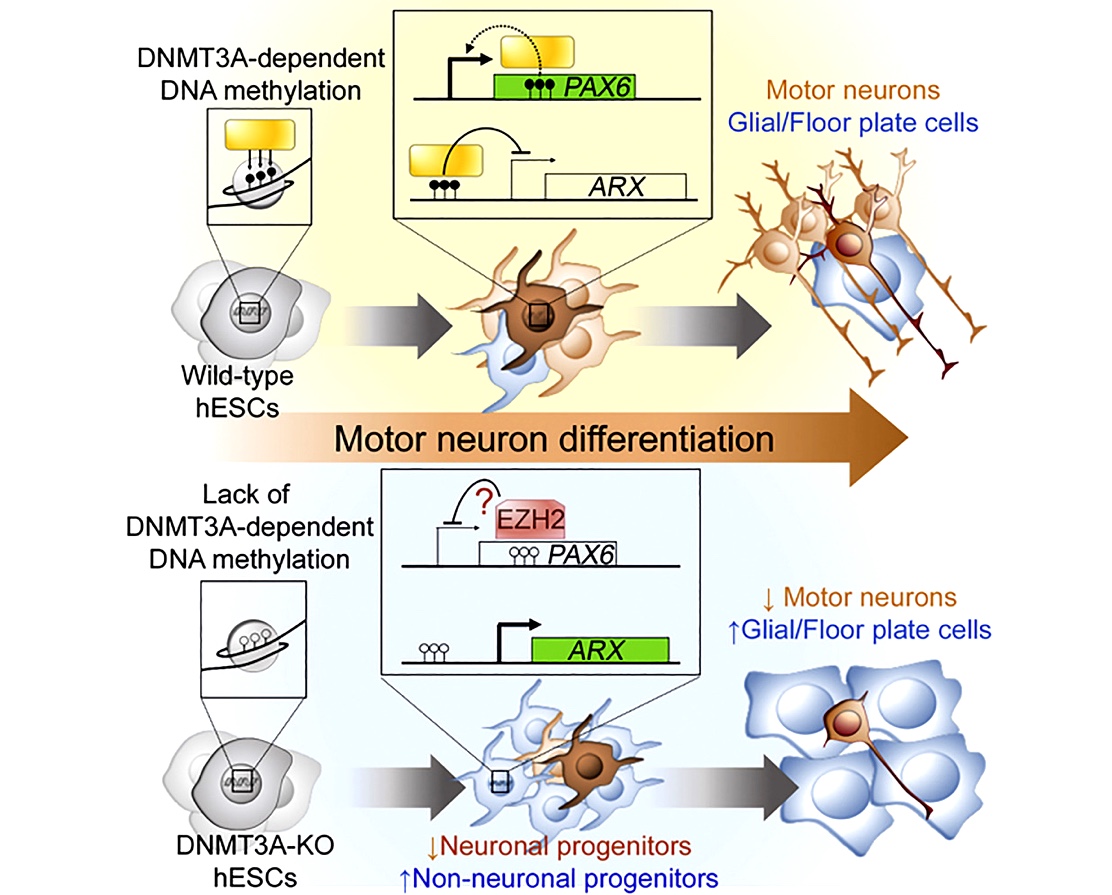
Developing protocols and technologies to improve iPSC-based models
We are focused on improving our ability to model neurological diseases using iPSC approaches. As an example, we seek to define the epigenetic mechanisms that govern the developmental trajectory of pluripotent stem cells towards their directed differentiation into postmitotic motor neurons to improve the fidelity of human motor neurons made in the lab (Ziller et al., Cell Stem Cell). More recently we reported the surprising finding that CRISPR/Cas9-edited iPSC lines exhibit a high incidence of large on-target re-arrangements that can be hard to detect (Simkin et al., Stem Cell Reports). We established a cost-efficient quality control strategy that successfully identified all edited clones with detrimental on-target events and could facilitate the integrity of iPSC-based studies. In another project we are working towards developing novel extracellular matrix (ECM) mimetic materials to culture iPSC-derived neural cells on synergistic cues derived from the chemical composition and molecular dynamics of the native ECM (Alvarez et al., Cell Stem Cell).
Publications
View a full list of our publications on Pubmed.
View the impact of our work on Google Scholar.
View representative publications below and a full list per year here.
Dissecting the Functional Consequences of De Novo DNA Methylation Dynamics in Human Motor Neuron Differentiation and Physiology
Ziller MJ, Ortega JA, Quinlan KA, Santos DP, Gu H, Martin EJ, Galonska C, Pop R, Maidl S, Di Pardo A, Huang M, Meltzer HY, Gnirke A, Heckman CJ, Meissner A, Kiskinis E
Cell Stem Cell. 2018 Apr 5 | PMID: 29551301
Dyshomeostatic modulation of Ca 2+-activated K + channels in a human neuronal model of KCNQ2 encephalopathy
Simkin D, Marshall KA, Vanoye CG, Desai RR, Bustos BI, Piyevsky BN, Ortega JA, Forrest M, Robertson GL, Penzes P, Laux LC, Lubbe SJ, Millichap JJ, George AL Jr, Kiskinis E
eLife. 2021 Feb 5 | PMID: 33544076
Nucleocytoplasmic Proteomic Analysis Uncovers eRF1 and Nonsense-Mediated Decay as Modifiers of ALS/FTD C9orf72 Toxicity
Ortega A, Daley ET, Kour S, Samani M, Tellez L, Smith HS, Hall EA, Esengul TY, Tsai T, Gendron TF, Donnelly CJ, Siddique T, Savas JN, Pandey UB and Kiskinis E
Neuron. 2020 Apr 8 | PMID: 32059759
Latest News
See our latest news and social interactions here.

For more news click here.
People
Our lab is currently comprised of 12 exceptionally talented, hard-working, and passionate individuals. These include, 5 postdoc fellows, 5 graduate students, a bioinformatics analyst, and a lab manager.
Click here to meet our team members.
Evangelos Kiskinis, PhD Principal Investigator
Assistant Professor of Neurology and Neuroscience, New York Stem Cell Foundation
Robertson Investigator, Scientific Director Stem Cell Core Facility
Evangelos was born in Thessaloniki, Greece. He moved to the UK when he was 19 and did his undergraduate studies at the University of Surrey and graduate work at Imperial College London.
In between, he spent a year in Basel, Switzerland working as a research trainee at Novartis Pharmaceuticals. Evangelos trained as a postdoctoral fellow in Kevin Eggan's lab at the Harvard Stem Cell Institute working on
harnessing the utility of stem cells to study and treat neurodegenerative disease. He has been the recipient of postdoctoral fellowships from the European Molecular Biology Organization (2008), the New York Stem Cell Foundation (2011)
and the Charles King Trust (2013). Evangelos moved to Northwestern University in January 2015 to head his own group, which focuses on studying neurological diseases using stem cell-based approaches.
At Northwestern, he also serves as the Director of the Stem Cell Core Facility. He enjoys spending his time with his young family as well as reading about his literary heroes that
include Corto Maltese and Hellboy.
Phone: 312-503-6039 | E-mail: evangelos.kiskinis[at]northwestern.edu

Lab Life
Click each photo, to view the respective gallery.
Join us
For inquires related to job opportunities and the research we do please contact Evangelos.
Phone: 312-503-6039 | E-mail: evangelos.kiskinis[at]northwestern.edu
Summer Internships
If you are interested in joining our lab for a summer internship please email us with a copy of your CV and a short cover letter explaining your interest in our lab.
Undergraduate Students
We are always looking for motivated and dedicated students who are willing to commit at least one year to the lab. Please email us with a copy of your CV and a short cover letter explaining your interest in our lab.
Masters Students
We are always looking for motivated and dedicated students who are willing to commit at least one year to the lab. Please email us with a copy of your CV and a short cover letter explaining your interest in our lab.
PhD Students
Prospective PhD students can join the lab only through Northwestern's Interdepartmental Neuroscience (NUIN) program, the Driskill Graduate Program (DGP) in Life Sciences, or the Medical Scientist Training Program (MSTP).
Post-Doctorate Fellows
We are accepting post-doctoral applications. Applicants should have a PhD with at least three years of proven research experience as well as at least one first author publication. Expertise in any of the following approaches: neurobiology, biochemistry, computational biology, single-cell RNA Sequencing, bioinformatics and molecular biology are highly favorable. Please email us with a copy of your CV, contact information for three references and a cover letter.
Information for ALS Patients
Clinical Trials
The Northeast ALS Consortium (NEALS) is an independent consortium of researchers who conduct clinical research in motor neuron diseases.
Find information on clinical trials for ALS.
The Dedicated ALS Clinic at Northwestern
The Lois Insolia ALS Clinic at the Les Turner ALS Center is dedicated to the total care and support of people with ALS, their families and caregivers.
The ALS Association
The ALS Association is a national non-profit organization fighting ALS on every front. By leading the way in global research,
helping people with ALS through a nationwide network of chapters, coordinating multidisciplinary care through certified clinical care centers, and fostering government partnerships,
the Association builds hope and enhances quality of life while aggressively searching for new treatments and a cure.
Contact Us
The lab is superbly located in the downtown Chicago area, a few minutes away from Lake Michigan,
the shopping and restaurants of Magnificent Mile, within the historical Montgomery Ward Memorial Building.
For general lab inquires please contact our lab manager Kevin at: Phone: 312-503-6126 | E-mail: kevin.smith@northwestern.edu
Address
Evangelos Kiskinis Lab | Northwestern UniversityFeinberg school of Medicine | Department of Neurology
Searle 6-450 | 320 East Superior Street
Chicago, IL 60611, USA


